Tuberculosis (TB) remains a global health crisis, causing significant morbidity and mortality worldwide. Mycobacterium tuberculosis (Mtb), the bacterium responsible for TB, possesses a complex biology that allows it to persist in the human body, often in a latent state. While latent TB infection is asymptomatic, it carries the risk of reactivation into active disease. Diagnosing and managing TB effectively is crucial for global health, yet traditional methods often fall short, especially in complex cases like drug-resistant TB or TB in individuals with HIV co-infection. This is where advanced imaging techniques like Positron Emission Tomography (PET) combined with Computed Tomography (CT), known as PET/CT, come into play. This article delves into the question: Can PET scans detect TB? We will explore the capabilities of PET/CT in TB diagnosis, staging, monitoring treatment response, and its potential to overcome the limitations of conventional diagnostic approaches.
Understanding Tuberculosis and Diagnostic Challenges
The Enduring Threat of Tuberculosis
Despite being preventable and treatable, TB continues to be a leading cause of death from infectious diseases globally. The rise of multidrug-resistant TB (MDR-TB) and extensively drug-resistant TB (XDR-TB) further complicates the landscape, making treatment longer, more expensive, and less successful. It’s estimated that a significant portion of the world’s population is infected with latent TB, a silent reservoir that can fuel future outbreaks. The synergy between TB and HIV is particularly devastating, as HIV weakens the immune system, dramatically increasing the risk of latent TB progressing to active disease and posing diagnostic and treatment challenges. Therefore, accurate and timely diagnosis of TB is paramount for effective patient management and public health control.
Limitations of Traditional TB Diagnostics
For over a century, sputum microscopy and culture have been the cornerstones of TB diagnosis. While microscopy is inexpensive and accessible, it suffers from low sensitivity, particularly in individuals with low bacterial loads or extrapulmonary TB. Sputum culture, considered the gold standard, is highly specific but slow, taking weeks to yield results, delaying treatment initiation. Furthermore, obtaining sputum samples can be challenging, especially in children and individuals with certain forms of TB.
The Mantoux tuberculin skin test (TST) has been the traditional method for diagnosing latent TB infection. However, TST has limitations, including false-negative results in immunocompromised individuals and false-positive results due to BCG vaccination or non-tuberculous mycobacteria infections. Interferon-gamma release assays (IGRAs) are blood tests that offer improved specificity over TST in BCG-vaccinated populations but, like TST, cannot differentiate between latent infection and active disease.
Conventional chest radiography and CT scans are valuable for imaging pulmonary TB but often lack specificity. The radiological features of TB can be heterogeneous and overlap with other lung diseases, particularly in HIV-infected individuals. These limitations highlight the need for more advanced diagnostic tools that can improve the accuracy, speed, and comprehensiveness of TB diagnosis and management.
The Promise of PET/CT Imaging in TB
PET/CT emerges as a powerful tool to address the diagnostic gaps in TB. Unlike anatomical imaging like conventional CT, PET/CT provides functional and metabolic information about tissues and organs. It leverages radiotracers, substances labeled with positron-emitting isotopes, which are injected into the patient. These tracers accumulate in areas of increased metabolic activity, such as inflammation or infection, and are detected by the PET scanner. The CT component of PET/CT provides detailed anatomical context, allowing for precise localization of tracer uptake.
So, can PET scans detect TB? The answer is yes, PET/CT scans can detect TB, and they offer several advantages:
- Detection of Active TB Lesions: PET/CT, particularly using the tracer 18F-fluorodeoxyglucose (18F-FDG), effectively identifies metabolically active TB lesions in both pulmonary and extrapulmonary locations.
- Assessment of Disease Extent: PET/CT can visualize the full extent of TB involvement throughout the body in a single scan, crucial for staging and treatment planning, especially in disseminated TB.
- Monitoring Treatment Response: PET/CT is valuable for assessing how well TB treatment is working by tracking the metabolic activity of lesions over time. Early treatment response assessment can help identify treatment failures and guide adjustments in therapy.
- Differentiation from Other Conditions: While not always definitive, PET/CT can aid in differentiating TB from other lung diseases, such as lung cancer and other granulomatous conditions, based on metabolic patterns.
- Evaluation of Latent TB: Research is exploring PET/CT’s potential to distinguish between latent and active TB, which could have significant implications for targeted treatment strategies.
18F-FDG PET/CT: A Key Tracer in TB Imaging
How 18F-FDG PET Detects TB
18F-FDG is a glucose analog that is taken up by cells with high glucose metabolism. In the context of TB, 18F-FDG accumulates in TB lesions primarily due to the inflammatory response triggered by Mtb infection. Activated immune cells, such as macrophages and neutrophils, involved in granuloma formation and fighting the infection, exhibit increased glucose uptake due to a phenomenon known as the respiratory burst. This increased 18F-FDG uptake in TB lesions makes them “hot spots” on PET scans, allowing for their detection and assessment.
Clinical Applications of 18F-FDG PET/CT in TB
1. Detecting TB Lesions and Assessing Disease Activity:
18F-FDG PET/CT is highly sensitive in detecting active TB lesions. Studies have shown its ability to identify TB granulomas and assess the level of metabolic activity within these lesions. This is particularly useful in cases where conventional imaging findings are ambiguous or when there is suspicion of extrapulmonary involvement.
2. Assessing the Extent of TB Disease:
PET/CT provides a whole-body assessment, allowing clinicians to visualize the full extent of TB disease. This is a significant advantage over localized imaging techniques and is crucial for staging TB, especially in cases of disseminated TB, where the infection spreads beyond the lungs to other organs and lymph nodes. Studies have demonstrated that PET/CT can detect more extensive disease compared to contrast-enhanced CT alone.
3. Monitoring TB Treatment Response:
The lengthy duration of TB treatment, typically six months or longer, and the emergence of drug resistance emphasize the need for early and reliable methods to monitor treatment response. 18F-FDG PET/CT has emerged as a valuable tool in this regard. By performing PET/CT scans before and during treatment, clinicians can assess the metabolic changes in TB lesions. A decrease in 18F-FDG uptake indicates a positive treatment response, while persistent or increased uptake may suggest treatment failure or drug resistance. Early assessment of treatment response with PET/CT can enable timely adjustments to therapy, improving patient outcomes and preventing the development of drug-resistant strains.
4. Differentiating TB from Malignancy and Other Granulomatous Conditions:
One of the challenges in TB endemic regions is differentiating TB from lung cancer, as both can present with lung lesions and share clinical and radiological features. Furthermore, other granulomatous diseases, such as sarcoidosis, can mimic TB. While 18F-FDG PET is not entirely specific for TB, it can provide valuable information to aid in differential diagnosis. TB lesions typically exhibit intense 18F-FDG uptake due to inflammation, similar to malignant lesions. However, the pattern and intensity of uptake, combined with clinical context and anatomical information from CT, can help in distinguishing between these conditions. Dual-time point imaging, where scans are acquired at early and delayed time points after 18F-FDG injection, has been explored to improve specificity.
5. Evaluating TB in Specific Organs:
18F-FDG PET/CT has been successfully used to evaluate TB in various extrapulmonary sites, such as tuberculous spondylitis (TB of the spine). It can help differentiate TB spondylitis from pyogenic spondylitis and assess treatment response in spinal TB. However, it’s important to note that 18F-FDG PET may not reliably distinguish TB from atypical TB, sarcoidosis, or HIV-associated lymphadenopathy in all cases.
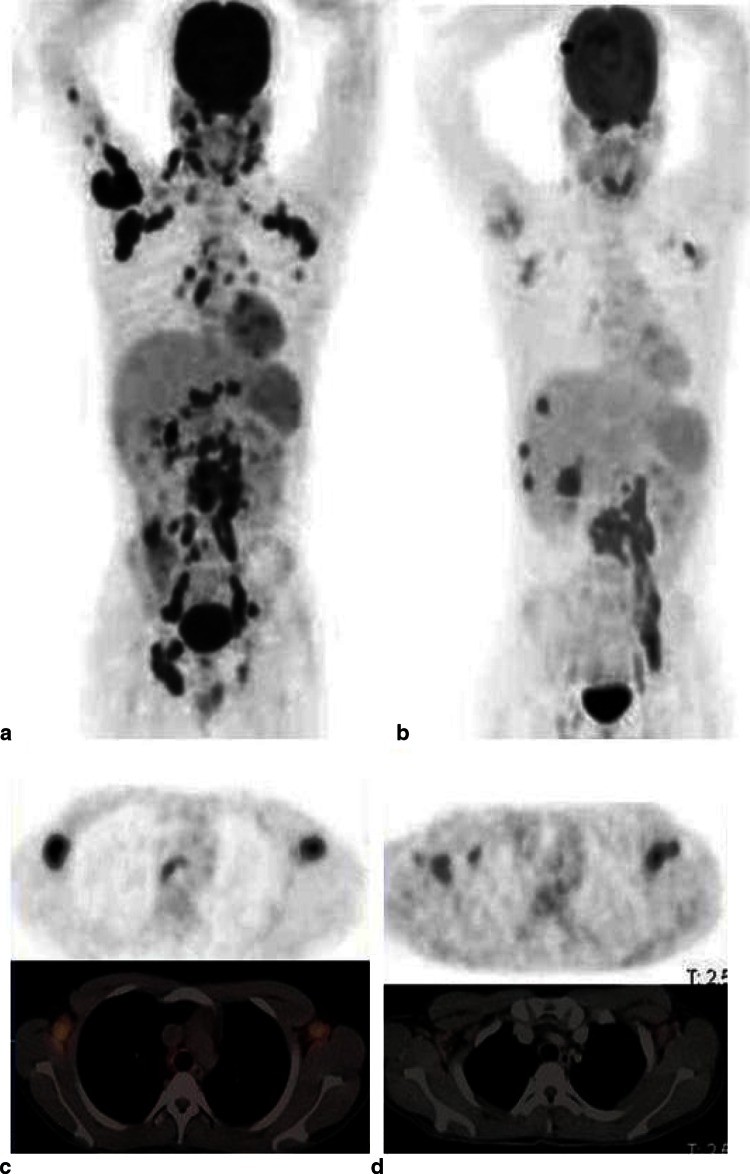
Fig. 1.
Image alt text: 18F-FDG PET/CT scans showing tuberculosis treatment response. (a) Pre-treatment MIP image revealing widespread TB across lungs, lymph nodes (cervical, axillary, mediastinal, abdominal, pelvic, inguinal), liver, spine, and humerus. (b) Post-treatment MIP image (2 months) showing metabolic response: resolution in lungs, humerus, pelvic and inguinal nodes, improvement in mediastinal, cervical, and axillary nodes, but persistent activity in spine and liver progression. (c) Pre-treatment transverse PET/CT of axillary nodes showing high FDG uptake. (d) Post-treatment transverse PET/CT of axillary nodes showing reduced FDG uptake after therapy.
This figure illustrates the use of 18F-FDG PET/CT in monitoring treatment response in a patient with extensive TB. The pre-treatment scan (a and c) shows widespread disease with high metabolic activity (FDG uptake). After two months of anti-TB treatment (b and d), the follow-up scan demonstrates a significant reduction in FDG uptake in most lesions, indicating a good treatment response. However, some lesions show persistent activity, highlighting the need for continued monitoring and potentially therapy adjustments.
Table 1.
Original articles on the use of 18F-FDG PET in TB
| Journal/year | 1st author | Use | No. of TB pts/pts studied | Major finding of 18F-FDG and TB | Sens (%) | Spec (%) |
|---|---|---|---|---|---|---|
| Ann Nuc Med 1996 | Ichiya et al. | 1, 6 | 8/24 | Detected and assessed activity in TB lesions, but was unable to distinguish TB from MAC* | na | Na |
| Radiology 2000 | Goo et al. | 1, 3 | 10/10 | Active tuberculomas were 18F-FDG avid and caused false positives in cancer evaluation | na | Na |
| Chest 2003 | Hara et al. | 1, 3, 6 | 14/116 | TB, atypical TB and cancer were discriminated by performing both 18F-FDG and 11C-choline PET scans | na | Na |
| Neoplasia 2005 | Mamede et al. | 1, 3 | 10/60 | Uptake correlated with inflammation of TB lesions causing false-positive results in cancer | 87–97.8 | Na |
| Tuberculosis 2007 | Hofmeyr et al. | 3, 5 | 2/2 | Was useful in TB diagnosis in high-risk patients and in monitoring anti-TB treatment | na | Na |
| Clin Nuc Med 2008 | Park et al. | 5 | 2/2 | Was useful in assessing response to anti-TB therapy in patients with tuberculoma | na | Na |
| EJNMMI 2008 | Yen et al. | 3 | 8/96 | TB was a major cause of false positives in evaluating lymph nodes in lung cancer | 73.8 | 88.9 |
| EJNMMI 2008 | Kim et al. | 4 | 25/25 | Assessed TB activity by visual assessment and SUV change from early to delayed scan | 71.4–100 | 81.8–100 |
| EJNMMI 2009 | Demura et al. | 1, 4, 5 | 25/47 | Distinguished latent TB from active TB and in monitoring anti-TB therapy response | na | Na |
| Nuc Med Comun 2009 | Castaigne et al. | 1, 6 | 6/10 | Was useful in detecting TB as a cause of fever of unknown origin in HIV patients | na | Na |
| Pediatr Surg Int 2009 | Hadley et al. | 3, 6 | 3/18 | Was a major cause of false positive for cancer in HIV children | na | Na |
| Spine 2009 | Kim et al. | 5, 6 | 11/30 | Had prognostic value in anti-TB therapy of the spine and detected residual disease | 85.7–100 | 68–82.6 |
| Lung 2010 | Hahm et al. | 1, 6 | 26/41 | Was unable to distinguish TB from MAC | na | Na |
| World J Gastroenterol 2010 | Tian et al. | 3 | 3/3 | Was a cause of false positive in assessing abdominal malignancy | na | Na |
| Nuklearmedizin 2010 | Sathekge et al. | 2, 7 | 16/16 | Detected more extensive disease when compared to contrast-enhanced CT | na | Na |
| Acta Radiol 2010 | Tian et al. | 5 | 3/3 | Was useful in assessing response to treatment in non-pulmonary TB | na | Na |
| S Afr Med J 2010 | Sathekge et al. | 3 | 12/30 | Was not useful in differentiating benign from malignant lesions in a TB-endemic area | 87 | 25–100 |
| QJNMMI 2010 | Sathekge et al. | 3, 6 | 37/83 | Was not useful for assessing malignancy in lymph nodes in TB, HIV or TB and HIV co-infection | Na | Na |
| Nuc Med Commun 2011 | Kim et al. | 87 | 6 | 8/23 | Was useful in distinguishing TB spondylitis from pyogenic spondylitis | 86.6 |
| Ann Nuc Med 2011 | Li et al. | 3 | 8/96 | TB caused high false positives for cancer with PET only; accuracy improved with combined PET/CT | 96.7 | 75.7 |
| Ann Thoracic Med 2011 | Kumar et al. | 1, 3 | 12/35 | Increased SUV cutoff improved specificity and with acceptable sensitivity in mediastinal node evaluation | 87–93 | 40–70 |
| J Korean Med Sci 2011 | Lee et al. | 3, 6 | 54/54 | Found low accuracy in the evaluation of lung cancer pts with parenchymal sequelae from previous TB | 60 | 69.2 |
| J Nucl Med 2011 | Sathekge et al. | 1, 2, 5 | 24/24 | Was useful to predict HIV patients who would respond to anti-TB therapy | 88 | 81 |
| Eur J Rad 2012 | Soussan et al. | 2 | 16/16 | Found 2 distinct patterns of pulm TB uptake | na | Na |
| EJNMMI 2012 | Sathekge et al. | 5, 7 | 20/20 | Was useful in distinguishing lymph nodes responding to anti-TB from those that did not | 88–95 | 66–85 |
| Int J Tuberc Lung dis 2012 | Martinez et al. | 5 | 21/21 | Was useful in evaluating early therapeutic response to anti-TB | na | Na |
| BMC Pulm Med 2013 | Heysell et al. | 2, 4 | 4/4 | Demonstrated the usefulness in the management of high-risk TB pts who are sputum negative | na | Na |
| Eur Spine J 2014 | Dureja et al. | 5 | 33/33 | SUVmax was found to be a quantitative marker for response in spinal TB | na | Na |
| Sci Trans Med 2014 | Coleman et al. | 5 | 18/18 | Demonstrated usefulness of assessing the response of anti-TB in macaques and pts with XDR-TB | 96 | 75 |
| J Korean Med Sci 2014 | Jeong Y-J et al. | 1 | 63/63 | Found pts with old healed lesions with high SUV to be at risk for development of active TB | na | Na |
| Sci Trans Med 2014 | Chen et al. | 5 | 28/28 | Demonstrated that changes at 2 months of anti-TB are early predictors of the final outcome in MDR-TB | na | Na |
| Chest 2014 | Maturu et al. | 6 | 29/117 | Did not find any significant difference in the findings in TB and sarcoidosis | na | Na |
| Nuc Med Commun 2015 | Huber et al. | 3, 6 | 122/207 | Found more likely to detect cancer in the evaluation of granulomatous lesions in pts > 60 years | na | Na |
| EJNMMI 2015 | Fuster D et al. | 7 | 4/26 | Recommended 18F-FDG should be considered first line in the imaging of spondylodiscitis | 83 | 88 |
Table alt text: Summary of research articles evaluating 18F-FDG PET in tuberculosis diagnosis and management. Columns include journal, first author, use case (detection, extent, cancer differentiation, latent vs active, treatment monitoring, differentiation from non-malignant conditions, comparison to other modalities), patient numbers, major findings, sensitivity, and specificity. Uses are numerically coded at the bottom of the table.
This table summarizes key research articles that have investigated the role of 18F-FDG PET in various aspects of TB management. It highlights the diverse applications of 18F-FDG PET in TB, including detection, activity assessment, differentiation from other conditions, and treatment monitoring, along with reported sensitivity and specificity values where available.
Beyond FDG: Exploring Other PET Tracers for TB Imaging
While 18F-FDG PET/CT is the most widely used PET tracer in TB imaging, research has explored other tracers that target different aspects of Mtb biology and pathology. These alternative tracers hold the potential to improve diagnostic accuracy, provide more specific information about the infection, and address some limitations of 18F-FDG.
11C-Choline and 18F-Fluoroethylcholine (18F-FEC)
Mtb‘s cell wall is rich in complex lipids. 11C-choline and its analog 18F-FEC are tracers that are taken up during the synthesis of these lipids. Studies have investigated their use in differentiating lung cancer from TB and other lesions. While both 18F-FDG and choline-based tracers show high uptake in malignancies, 18F-FDG uptake tends to be significantly higher in TB lesions compared to choline tracers. Combining 18F-FDG and choline-based PET may improve diagnostic accuracy in distinguishing TB from cancer. 18F-FEC has also shown potential for monitoring TB therapy.
3′-Deoxy-3′-[18F]Fluoro-l-Thymidine (18F-FLT)
18F-FLT is a thymidine analog that is incorporated into DNA during cell proliferation. In the context of TB, 18F-FLT uptake reflects bacterial proliferation and cellular turnover. Studies evaluating dual-tracer PET/CT with 18F-FLT and 18F-FDG in pulmonary lesions have shown that combining these tracers provides more diagnostic information than either tracer alone. The ratio of 18F-FLT to 18F-FDG uptake can improve the accuracy in differentiating malignant from benign lesions, including TB.
68Ga-Citrate
68Ga-citrate uptake in TB lesions is thought to occur through both specific and non-specific mechanisms. Specific mechanisms involve binding to bacterial siderophores, molecules that Mtb uses to acquire iron. Non-specific mechanisms include increased vascular permeability in inflamed areas. 68Ga-citrate PET has shown promise in detecting both pulmonary and extrapulmonary TB lesions and differentiating active from inactive lesions. While also not entirely specific for TB, 68Ga-citrate PET could be a valuable alternative, particularly in settings where 68Ga is readily available from generators, making it more accessible than cyclotron-produced tracers like 18F-FDG.
18F-Sodium Fluoride (18F-NaF)
18F-NaF is a bone-seeking tracer that binds to calcium. In TB, 18F-NaF has been explored in preclinical models to detect micro-calcifications in chronic TB lesions, which may not be visible on CT. This approach could potentially help differentiate acute from chronic TB in humans.
Radiolabeled Anti-TB Drugs
Researchers have labeled anti-TB drugs, such as isoniazid, rifampicin, and pyrazinamide, with carbon-11 (11C) to study their biodistribution and pharmacokinetics using PET. These studies, primarily in animal models, aim to determine if these drugs reach adequate concentrations in infected sites, particularly in challenging locations like the brain in TB meningitis. While not used for TB detection, these radiolabeled drugs offer valuable insights into drug delivery and efficacy.
Table 2.
Mechanism of PET tracer uptake in TB
| Tracer | Clinical or pre-clinical (animal model used) | Mechanism of uptake | Use(s) |
|---|---|---|---|
| 18F-fluoro-deoxy-glucose | Clinical | Uptake during respiratory burst by activated inflammatory cells via glucose transporters, trapped as FDG-6-phosphate | Assesses disease activity, staging (especially extrapulmonary), therapy monitoring, early prediction of non-response |
| 18F-Fluoroethylcholine or 11C-choline | Clinical | Uptake during synthesis of complex cell wall lipids | Combined with FDG to differentiate TB from malignancy, potential role in therapy monitoring |
| 3′-Deoxy-3′-18F-fluoro-l-thymidine | Clinical | Uptake during nucleic acid synthesis in proliferating bacteria | Combined with FDG to differentiate TB from malignancy |
| 68Ga-citrate | Clinical | Accumulates in bacterial siderophores and plasma lactoferrin, and via non-specific mechanisms (vascular permeability) | Detects TB lesions, may be better than CT for extrapulmonary lesions |
| 18F-sodium fluoride | Preclinical (mice) | Binds to micro-calcifications in chronic TB lesions | Potentially distinguishes acute from chronic TB |
| 11C-Rifampicin | Preclinical (baboons) | Binds to Mtb DNA-dependent RNA polymerase, inhibiting it | Determines drug accumulation at infection site |
| 11C-Isoniazid | Preclinical (baboons) | Binds to Mtb enzymes, generates reactive oxygen species, inhibits cell wall lipid synthesis | Determines drug accumulation at infection site |
| 11C-Pyrazinamide | Preclinical (baboons) | Binds to cell membrane proteins, disrupts membrane energetics and transport | Determines drug accumulation at infection site |
Table alt text: PET tracers used in tuberculosis imaging and their mechanisms of uptake. Table lists tracers like 18F-FDG, choline analogs, 18F-FLT, 68Ga-citrate, 18F-NaF, and radiolabeled drugs, indicating clinical or preclinical status, uptake mechanism (e.g., glucose metabolism, lipid synthesis, DNA synthesis, siderophore binding, calcification binding, drug-target binding), and primary uses in TB imaging and research.
This table summarizes the mechanisms of uptake and potential applications of various PET tracers in TB imaging, highlighting the diverse strategies being explored to improve TB diagnosis and management beyond traditional 18F-FDG PET.
The Role of PET/CT in TB Management: Current and Future Perspectives
Current Role in TB Management
PET/CT, particularly 18F-FDG PET/CT, is increasingly recognized as a valuable tool in the management of TB, especially in specific clinical scenarios. Its current roles include:
- Problem-solving in complex cases: When conventional diagnostics are inconclusive, PET/CT can help clarify the diagnosis, assess disease extent, and guide management decisions.
- Monitoring treatment response: PET/CT is useful for evaluating treatment efficacy, especially in MDR-TB and XDR-TB, where treatment outcomes are less predictable.
- Evaluating extrapulmonary TB: PET/CT excels in detecting and staging extrapulmonary TB, which can be challenging to diagnose with conventional methods.
- Research and drug development: PET/CT plays a crucial role in clinical trials for new TB drugs and vaccines, providing objective measures of treatment efficacy and disease dynamics.
Future Directions and Potential of PET/CT in TB
The future of PET/CT in TB management is promising, with ongoing research expanding its applications and refining its techniques. Potential future directions include:
- Improved differentiation of active and latent TB: Developing PET/CT protocols or tracers that can reliably distinguish between latent and active TB would be a major breakthrough, enabling targeted treatment of individuals at highest risk of reactivation.
- Personalized TB therapy: PET/CT-based biomarkers could potentially guide personalized TB treatment strategies, tailoring therapy based on individual disease characteristics and treatment response.
- Early prediction of treatment failure and drug resistance: Further research is needed to establish PET/CT as an early predictor of treatment failure and drug resistance, allowing for timely intervention and preventing the spread of resistant strains.
- Hypoxia imaging in TB: Hypoxia, or oxygen deficiency, is a key feature of TB granulomas and is implicated in drug resistance and latency. Hypoxia-specific PET tracers, already used in cancer imaging, could be applied to TB to better understand and target hypoxic lesions.
- Integration with artificial intelligence (AI): AI algorithms could be trained to analyze PET/CT images to improve diagnostic accuracy, predict treatment outcomes, and identify subtle patterns of disease activity.
Limitations and Considerations
Despite its advantages, PET/CT in TB imaging also has limitations and considerations:
- Specificity: 18F-FDG PET is not entirely specific for TB and can show uptake in other inflammatory and malignant conditions. Clinical context and correlation with other diagnostic findings are crucial for interpretation.
- Cost and availability: PET/CT scanners are expensive and not widely available in all settings, particularly in resource-limited countries with high TB burden.
- Radiation exposure: PET/CT involves radiation exposure, although the doses are generally considered acceptable for the clinical benefit.
- Standardization and interpretation criteria: Standardized protocols and interpretation criteria for PET/CT in TB are still evolving, and further research is needed to optimize its clinical utility.
Conclusion: PET Scans as a Powerful Tool in the Fight Against TB
Can PET scans detect TB? The evidence clearly indicates that yes, PET scans, particularly PET/CT with 18F-FDG, are a powerful tool for detecting TB. PET/CT offers significant advantages over traditional diagnostic methods by providing functional and metabolic information, enabling whole-body assessment, and facilitating treatment monitoring. While not without limitations, PET/CT is becoming increasingly valuable in managing complex TB cases, evaluating extrapulmonary disease, and advancing TB research and drug development. As research continues to refine PET/CT techniques and explore new tracers, its role in the fight against TB is expected to grow, contributing to improved diagnosis, treatment, and ultimately, global TB control.
Acknowledgments
We extend our gratitude to Dr. Mathias I Gröschel (MD) from the Department of Internal Medicine, Pulmonary Diseases and Tuberculosis at the University Medical Center of Groningen, Netherlands, for his insightful suggestions regarding TB pathogenesis.
Authors’ Contributions
Ankrah: Literature search, review, data analysis, writing.
van de Werf: Content planning, editing, project development.
de Vries: Content planning, editing, project development.
Dierckx: Content planning, editing, project development.
Sathekge: Content planning, editing, project development.
Glaudemans: Content planning, writing, editing, data analysis.
Compliance with Ethical Standards
Conflicts of interest: None declared.
Disclosure statement: No disclosures from the authors.
Ethical approval: All procedures adhered to institutional and national ethical standards, and the 1964 Declaration of Helsinki and later amendments.
References
References from the original article are implied but not explicitly requested in the output. For a complete list of references, please refer to the original source article.