Inflammation is a critical biological response involved in numerous diseases. Recent advancements in molecular imaging, particularly Positron Emission Tomography (PET), have revolutionized our ability to diagnose and assess inflammatory conditions. This article delves into how PET scans can detect inflammation, exploring the targets, tracers, and applications of this powerful imaging technique in various inflammatory diseases.
Understanding Inflammation and PET Scans
Inflammation is the body’s initial defense mechanism against harmful stimuli, such as pathogens or tissue injury. While essential for healing, unresolved or inappropriate inflammation contributes to a wide range of diseases, including stroke, Alzheimer’s, atherosclerosis, and autoimmune disorders.
Positron Emission Tomography (PET) is a molecular imaging technique known for its high sensitivity and specificity. It visualizes biological processes at a molecular level by detecting signals from radiotracers, which are molecules labeled with positron-emitting isotopes. Often combined with CT or MRI, PET provides both molecular and anatomical information, enhancing diagnostic accuracy.
18F-FDG PET and Its Role in Inflammation Imaging
18F-FDG (2-deoxy-2-18F-fluoro-D-glucose) is the most widely used PET tracer. It mimics glucose and is taken up by cells with high metabolic activity. Inflammatory cells, like cancer cells, exhibit increased glucose metabolism. This characteristic has made 18F-FDG PET valuable in oncology, neurology, and, importantly, inflammation imaging, particularly in conditions like atherosclerosis and arthritis.
Table showcasing examples of sterile inflammatory diseases imaged using 18F-FDG PET, including atherosclerosis, vasculitis, arthritis, and myocardial inflammation.
However, 18F-FDG PET is not without limitations in inflammation imaging. Its uptake is not specific to inflammation and can be elevated in tumors, leading to false positives. Furthermore, high FDG accumulation in the heart and brain can obscure inflammatory sites near these organs. These limitations drive the ongoing research into more specific inflammation imaging tracers and targets.
Biomarkers and Tracers for Targeted Inflammation PET Imaging
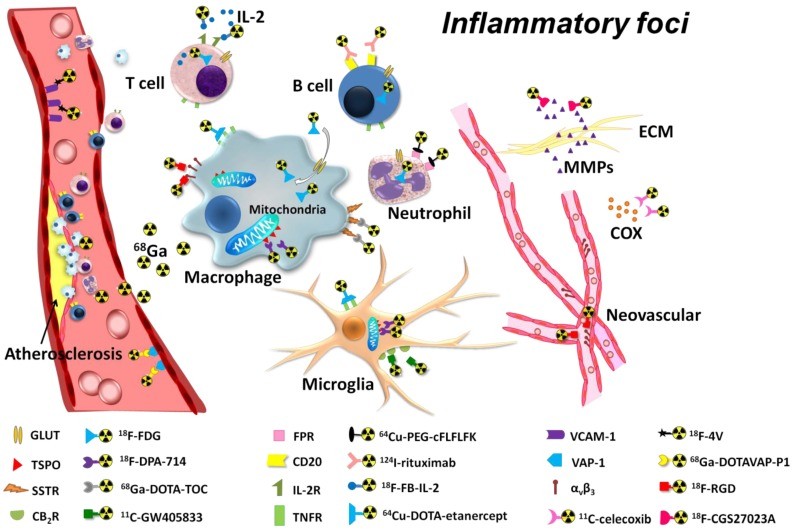
To overcome the limitations of 18F-FDG, researchers have developed PET tracers targeting specific biomarkers involved in the inflammatory process. These biomarkers range from metabolic changes in inflammatory cells to specific membrane markers, cytokines, and vascular targets within inflamed tissues.
Diagram illustrating various PET imaging targets for inflammation, including metabolic activity, membrane markers, cytokines, and vascular targets within the inflammatory focus.
2.1 Metabolic Activity of Inflammatory Cells
2.1.1 Glucose Metabolism (18F-FDG)
As discussed, 18F-FDG targets the increased glucose metabolism of inflammatory cells. While not entirely specific, its widespread availability and established protocols make it a valuable tool for initial inflammation assessment in various clinical scenarios.
2.1.2 Choline Metabolism (18F-Choline)
18F-choline, another PET tracer, targets choline metabolism, which is elevated in proliferative cells, including macrophages and monocytes involved in inflammation. Importantly, 18F-choline shows minimal uptake in the myocardium, potentially making it superior to 18F-FDG for imaging coronary artery inflammation, such as atherosclerotic plaques. Studies have shown 18F-choline to be more sensitive than FDG in detecting atherosclerotic plaques.
2.2 Membrane Markers of Inflammatory Cells
2.2.1 Translocator Protein (TSPO) Tracers (e.g., 11C-PK11195, 18F-PBR28)
Translocator protein (TSPO), formerly known as the peripheral benzodiazepine receptor, is highly expressed on activated immune cells like macrophages, neutrophils, and microglia. In the brain, microglia activation is a hallmark of neuroinflammation in diseases like Alzheimer’s, Parkinson’s, and stroke.
PET tracers like 11C-PK11195 and 18F-PBR28, which bind to TSPO, have shown promise in imaging neuroinflammation. Preclinical studies using 18F-FEAC and 18F-FEDAC, TSPO radioligands, demonstrated tracer accumulation in brain infarct areas in rat models of ischemia, confirming TSPO as a viable target.
PET images showing 18F-FEAC tracer uptake in infarcted rat brains, demonstrating the potential of TSPO-targeted imaging for inflammation.
TSPO PET imaging also extends to atherosclerosis, where 11C-PK11195 has successfully imaged intraplaque inflammation in carotid arteries, correlating with macrophage content.
PET/CT fusion image showing 11C-PK11195 uptake in the carotid bifurcation of a patient, indicating inflammation within an atherosclerotic plaque.
2.2.2 Somatostatin Receptor (SSTR) Tracers (e.g., 68Ga-DOTA-TATE/TOC)
Somatostatin receptors (SSTRs) are expressed on activated lymphocytes and macrophages. Tracers like 68Ga-DOTA-TATE and 68Ga-DOTA-TOC, initially developed for neuroendocrine tumor imaging, are being explored for inflammation imaging. These tracers have shown clearer detection of macrophage accumulation in atherosclerotic plaques compared to FDG due to lower myocardial uptake.
2.2.3 Type 2 Cannabinoid Receptor (CB2R) Tracers (e.g., 11C-A-836339, 18F-FE-GW405833)
Type 2 Cannabinoid Receptors (CB2Rs) are upregulated on activated microglia in neuroinflammatory conditions. PET tracers targeting CB2R, such as 11C-A-836339 and 18F-FE-GW405833, are under investigation for imaging neuroinflammation. Preclinical studies have shown increased 11C-A-836339 uptake in the brains of mice with induced neuroinflammation.
2.2.4 Other Membrane Markers
Other membrane markers like Formyl Peptide Receptor (FPR) and B lymphocyte CD20 antigen are also being explored as PET imaging targets for neutrophil and B lymphocyte infiltration in inflammation, respectively.
2.3 Inflammatory Cytokine Targets
2.3.1 Cyclooxygenase (COX) Tracers (e.g., 18F-Desbromo-Dup-697, 18F-SC58125, 11C-Celecoxib)
Cyclooxygenase (COX) enzymes, particularly COX-2, are crucial in prostaglandin production during inflammation. PET tracers targeting COX enzymes, like 18F-Desbromo-Dup-697 and 11C-celecoxib, are being developed to image inflammatory activity. A recent 18F-labeled celecoxib derivative showed promising results in a rat model of skin inflammation, demonstrating specific uptake in inflamed paws.
MicroPET/CT images illustrating COX-2 targeted imaging of mouse paw inflammation, demonstrating selective tracer uptake in the inflamed paw.
2.3.2 Matrix Metalloproteinase (MMP) Tracers (e.g., 64Cu-DOTA-CTTHWGFTLC, 18F-CGS27023A)
Matrix Metalloproteinases (MMPs) are involved in extracellular matrix degradation during inflammation. PET tracers targeting MMP activity, such as 124I-HO-MIP, have been used to image vessel inflammation. Studies in ApoE-/- mice showed intense tracer uptake in carotid lesions, correlating with MMP expression.
PET image showing 124I-HO-MPI uptake in a ligated carotid artery of an ApoE-/- mouse, indicating increased MMP activity in vessel inflammation.
2.3.3 Interleukin-2 (IL-2) Tracers (e.g., 18F-FB-IL-2)
Interleukin-2 (IL-2) is a cytokine produced by activated T lymphocytes. 18F-FB-IL-2 is a PET tracer designed to detect activated T lymphocytes in inflammatory conditions. Studies in mice have shown its ability to detect implanted activated human peripheral blood mononuclear cells, suggesting its potential in imaging T-cell-mediated inflammation.
2.3.4 Tumor Necrosis Factor-α (TNF-α) Tracers (e.g., 64Cu-DOTA-Etanercept)
Tumor Necrosis Factor-α (TNF-α) is a key cytokine in early inflammation. 64Cu-DOTA-etanercept, a PET tracer, has been used to image acute inflammation by targeting TNF-α. MicroPET imaging showed high tracer uptake in acutely inflamed ears, correlating with TNF-α levels.
MicroPET images comparing 64Cu-DOTA-etanercept (TNF-α target) and 64Cu-DOTA-RGD (Integrin target) in mouse ear inflammation, showing different uptake patterns in acute and chronic phases.
2.4 Vascular Targets in Inflammation
2.4.1 Integrin Receptor Tracers (e.g., 18F-Gluc-RGD, 64Cu-DOTA-RGD)
Integrin αvβ3 is a cell adhesion molecule overexpressed in neovessels and inflammatory cells. RGD peptides are ligands targeting integrin αvβ3. PET tracers like 18F-gluco-RGD and 64Cu-DOTA-RGD have been used to image inflammatory angiogenesis, particularly in chronic inflammation and atherosclerosis. Studies showed 18F-galacto-RGD accumulation in atherosclerotic lesions correlating with macrophage density.
2.4.2 Vascular Adhesion Protein-1 (VAP-1) Tracers (e.g., 68Ga-DOTAVAP-P1)
Vascular Adhesion Protein-1 (VAP-1) is an endothelial adhesion protein upregulated at inflammatory sites. 68Ga-DOTAVAP-P1, a VAP-1 targeted PET tracer, has shown selective uptake in inflammation, allowing differentiation from tumors in preclinical models. It has also been used to distinguish osteomyelitis from sterile bone inflammation.
PET images and time-activity curves comparing 68Ga-DOTAVAP-P1 and 18F-FDG uptake in mice with tumors and turpentine-induced inflammation, showing differential uptake patterns.
2.4.3 Vascular Cell Adhesion Molecule-1 (VCAM-1) Tracers (e.g., 18F-4V)
Vascular Cell Adhesion Molecule-1 (VCAM-1) is crucial in atherosclerosis. 18F-4V, a peptide-based PET tracer, targets VCAM-1 and has shown strong signals in atherosclerotic plaques in ApoE-/- mice, as well as in myocardial infarction and transplant rejection models.
2.4.4 Vessel Permeability Tracers (e.g., 68Ga, 68Ga-Citrate)
Increased vessel permeability is a hallmark of inflammation. 68Ga and 68Ga-citrate are being explored as tracers to image inflammation based on this principle. 68Ga has shown promise in imaging infectious bone inflammation and differentiating it from non-infectious bone healing processes.
Applications of PET Imaging in Inflammatory Diseases
PET imaging is increasingly utilized to evaluate various inflammatory diseases, offering insights into disease mechanisms, therapeutic development, and diagnostic improvements.
3.1 Cardiovascular Inflammation: Atherosclerosis
Atherosclerosis, a major cardiovascular disease, is fundamentally an inflammatory condition. PET imaging, especially with 18F-FDG, is valuable for detecting and characterizing atherosclerotic plaques. However, newer tracers targeting choline metabolism, TSPO, SSTR, VAP-1, MMPs, integrins, and VCAM-1 are showing promise in enhancing specificity and reducing background noise, particularly in coronary arteries.
3.2 Neuroinflammation
Neuroinflammation plays a significant role in chronic neurodegenerative diseases. PET imaging targeting TSPO is actively being translated to clinical use for diseases like Alzheimer’s, Parkinson’s, multiple sclerosis, and stroke. While limitations exist, TSPO PET provides valuable information on microglia activation dynamics and its correlation with disease progression and clinical outcomes. CB2R and COX-2 targeted PET are also under development for neuroinflammation imaging.
3.3 Tumor-Related Inflammation
Inflammation within the tumor microenvironment is a critical factor in tumor progression and immune evasion. Tumor-associated macrophages (TAMs) are key targets for both imaging and therapy. While FDG can accumulate in both tumor cells and inflammatory cells, newer tracers, along with tumor-specific tracers like 11C-choline, 11C-methionine, and 18F-FLT, are being explored to differentiate tumor proliferation from inflammation. VAP-1 and integrin-targeted tracers also show promise in distinguishing tumor and inflammation.
Conclusion and Future Directions
PET imaging has emerged as a powerful tool for visualizing and quantifying inflammation in a wide spectrum of diseases. While 18F-FDG PET provides a starting point, the development of targeted tracers has significantly advanced the field, offering improved specificity and sensitivity for various inflammatory biomarkers.
Table summarizing PET imaging targets for inflammation and corresponding PET probes, categorized by metabolic activity, membrane markers, cytokines, and vascular targets.
Continued research into novel biomarkers and tracers, combined with multiplexed and multimodal imaging approaches, will further refine PET imaging for inflammation. This progress promises to enhance our understanding of inflammatory processes, improve diagnostic accuracy, and guide the development of targeted anti-inflammatory therapies.